Clinical Education
On-site and virtual clinical nurse education
Our team of Clinical Nurse Liaisons has specialization in medication administration practices and are available for in-person and virtual support. We want clinicians to be comfortable and confident using Simplist®.

Simplist MicroVault Instructions for Use Video
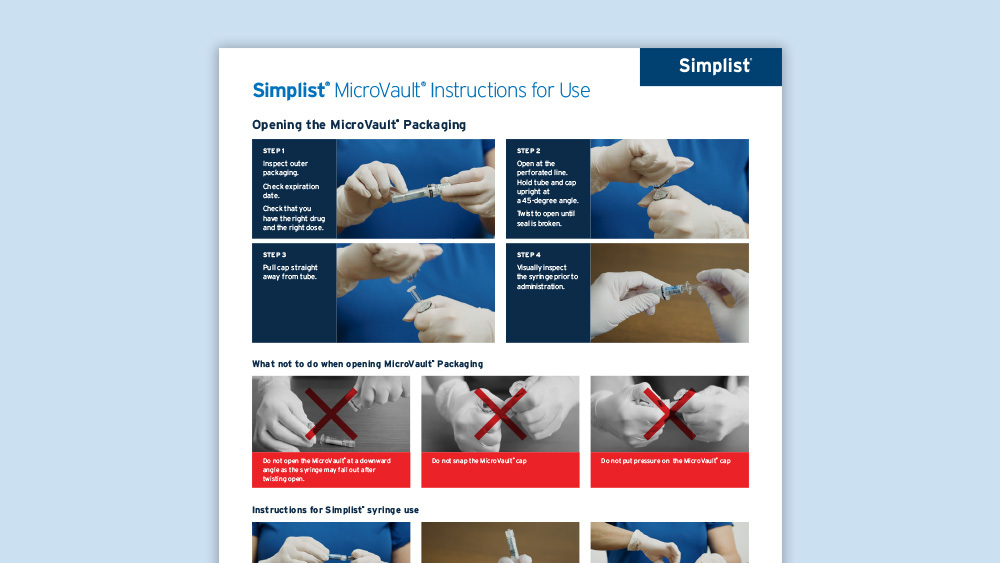
Simplist MicroVault Instructions for Use Poster

Case Studies
Explore the experiences of various healthcare institutions and professionals who have integrated Simplist syringes into their daily practices. From reducing medication waste to streamlining workflow, the case studies delve into the multifaceted benefits of using these prefilled syringes.
Pharmacy Storage Optimization
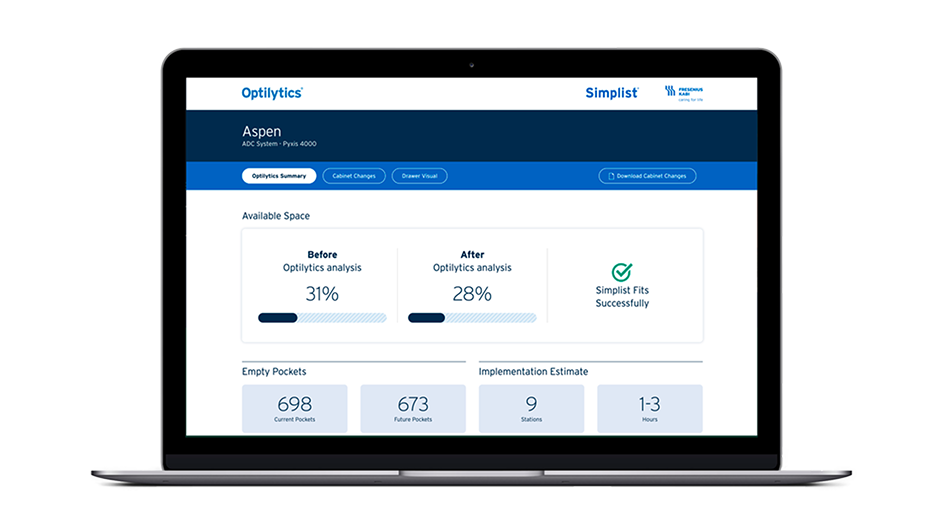
Optilytics®
- All Optilytics Simplist reports are complimentary
- Removes manual analysis of finding space for Simplist
- Provides directions to efficiently implement Simplist
- Optimize storage of currently loaded Simplist
Opioid Stewardship
We are committed to supporting leadership efforts for implementing hospital-wide opioid stewardship.
References
The Third Consensus Conference on the Safety of Intravenous Drug Delivery Systems was convened to evaluate the benefits and risks of available systems and assess ongoing threats to the safety of intravenous drug delivery.*
These guidelines are based on a patient-centric, team-based model of care created by leaders within the American Society of Anesthesiologists to help meet the demands of a rapidly approaching health care paradigm.
These care pathways form an integrated continuum, as the patient moves from home through the pre-hospital/pre-admission, preoperative, intraoperative, and postoperative phases of surgery and home again.
This article describes the rationale for use of multimodal analgesia and discusses non-opioid medications that can be used as part of a multimodal approach to postoperative pain relief.
These minimum standard guidelines are intended to serve as a basic guide for the provision of pharmacy services in hospitals. These guidelines outline a minimum level of services that most hospital pharmacy departments should consistently provide.
The purpose of these guidelines is to provide guidance to health systems on planning for and implementing best practices when establishing a comprehensive Controlled Substance Diversion Prevention Program.
This article discusses the financially significant costs associated with wasting both the product and the valuable time of a skilled workforce and ways to reduce this financial burden on our health-systems.*
This study suggests that careful selection of vial size has the potential to modify perioperative dosing practices and possibly reduce opioid use.
In an effort to ensure clinicians are clear about CDC guidelines, the Agency is restating its position on the use of single-dose/single-use vials and also seeks to dispel inaccuracies being disseminated to healthcare providers.
The ISMP Guidelines for Safe Practice of Adult IV Push Medications were developed to help healthcare facilities standardize the safe administration of parenteral IV push medications and prevent unsafe practices and at-risk behaviors associated with IV push administration of adult medications.*
The Joint Commission’s requirements outline a multi-level approach to pain management to help frontline staff and clinicians deliver safe, individualized pain care.
The tool is composed of four modules designed to help hospitals in assessing the challenges and barriers and prioritizing and developing corrective action plans to improve the safety of injectable medications.
In this article experts weigh in on what nurses need to know about IV Push drug safety.*
The purpose of this article is to review the existing literature and to assess the incidence and nature of errors related to the unnecessary dilution of RTA intravenous (IV) push medications in the inpatient clinical setting*
The use of manufacturer-prepared RTA prefilled syringes can play an important role in simplifying processes and reducing errors and related potential patient harm especially in high-stress critical situations, such as the COVID-19 pandemic.
Downloads
Simplist Product Catalog

Simplist MicroVault Brochure

Simplist MicroVault Instructions for Use Poster
Adenosine Product Family Information Card

Dexamethasone Product Family Information Card

Diazepam Product Family Information Card

Dilaudid Product Family Information Card
DiphenhydrAMINE Product Family Information Card
Fentanyl Product Family Information Card
Glycopyrrolate Product Family Information Card

Glycopyrrolate 0.6 mg per 3 mL Product Information Card
Heparin Product Family Information Card

Midazolam Product Family Information Card
Morphine Product Family Information Card
Neostigmine Product Family Information Card
Palonosetron Product Family Information Card
Package Inserts
- Adenosine Injection, USP
- Dexamethasone Sodium Phosphate Injection, USP (4 mg per 1 mL)
- Dexamethasone Sodium Phosphate Injection, USP (10 mg per 1 mL)
- Dilaudid® HYDROmorphone HCI Injection, USP*
- DiphenhydrAMINE Hydrochloride Injection, USP
- Fentanyl Citrate Injection, USP*
- Glycopyrrolate Injection, USP (0.2 mg per 1 mL and 0.4 mg per 2 mL)
- Glycopyrrolate Injection, USP (0.6 mg per 3 mL)
- Heparin Sodium Injection, USP (5,000 USP units per 0.5 mL)
- Heparin Sodium Injection, USP (5,000 USP units per 1 mL)
- Ketorolac Tromethamine Injection, USP*
- Midazolam Injection, USP*
- Morphine Sulfate Injection, USP*
- Neostigmine Methylsulfate Injection, USP
- Ondansetron Injection, USP
- Palonosetron Hydrochloride Injection
*Indicates Black Box Warning product