We manufacture high-quality, essential, life-saving medicines
Simplist® ready-to-administer prefilled syringes are manufactured by Fresenius Kabi, which operates 90 state-of-the-art manufacturing plants and R+D centers globally.
We have over 100 years of history supplying healthcare providers with over 500 million units annually of the generic injectable medications they need to provide life-saving care.
Today, 99% of our Simplist prefilled syringes are proudly formulated, filled and packaged right here in America.
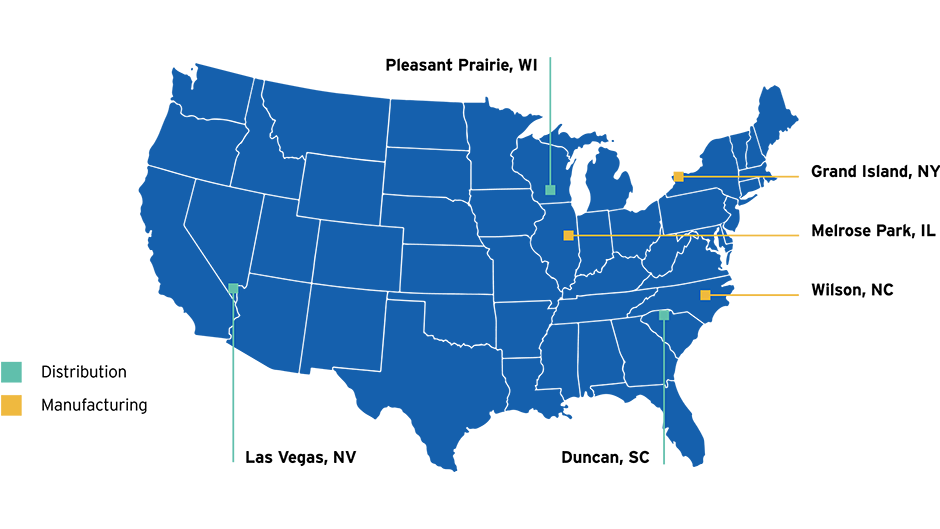
Simplist syringes are produced using a fully-automated state-of-the-art closed system in FDA-registered manufacturing plant in Wilson, North Carolina.

Wilson, North Carolina
Remaining Simplist prefilled syringes are produced in Graz, Austria

Graz, Austria
Our $1 Billion investment includes significant expansion of our U.S. supply chain infrastructure
This model helps essential drugs reach any location in the contiguous U.S. within 48 hours.
We continue to invest in manufacturing capabilities to support portfolio growth and long-term supply outlook.
Manufacturer prepared syringes eliminate the need for compounding
Compounded drugs are not FDA-approved. Drugs compounded by an outsourcing facility can qualify for exemptions from FDA approval requirements and the requirement to label products with adequate directions for use, but not from current good manufacturing practice (CGMP) requirements.1
Compounded drugs can serve an important medical need for patients, but they do not have the same safety, quality, and effectiveness assurances as approved drugs.2 Unnecessary use of compounded drugs unnecessarily exposes patients to potentially serious health risks.2
Benefits of Simplist ready-to-administer (RTA) prefilled syringes include:
- cGMP + FDA-approved
- Manufacturer prepared
- 24- or 36-month shelf life
- Clear and distinct labeling
Explore our portfolio.
We’re committed to providing ready-to-administer options that suit your needs. That’s why we’ve started with common concentrations of frequently-used medications. As we continue to expand, we’ll be able to offer even more options.
Explore products