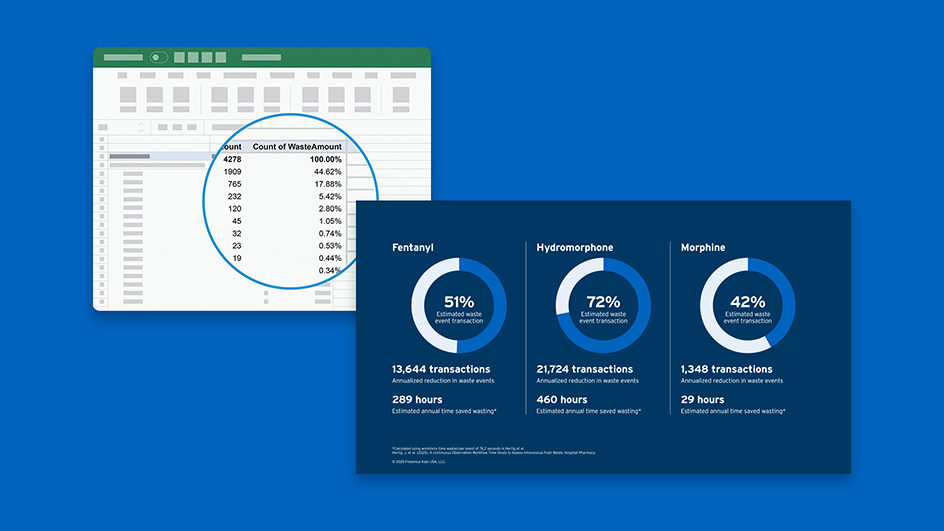
Succinylcholine Chloride Injection, USP
IMPORTANT SAFETY INFORMATION
WARNING: VENTRICULAR DYSRHYTHMIAS, CARDIAC ARREST, AND DEATH FROM HYPERKALEMIC RHABDOMYOLYSIS IN PEDIATRIC PATIENTS
- Acute rhabdomyolysis with hyperkalemia followed by ventricular dysrhythmias, cardiac arrest, and death has occurred after the administration of succinylcholine to apparently healthy pediatric patients who were subsequently found to have undiagnosed skeletal muscle myopathy, most frequently Duchenne muscular dystrophy.
- When a healthy appearing pediatric patient develops cardiac arrest within minutes after administration of Succinylcholine Chloride Injection, not felt to be due to inadequate ventilation, oxygenation or anesthetic overdose, immediate treatment for hyperkalemia should be instituted. In the presence of signs of malignant hyperthermia, appropriate treatment should be instituted concurrently.
- Reserve the use of Succinylcholine Chloride Injection in pediatric patients for emergency intubation or instances where immediate securing of the airway is necessary, e.g., laryngospasm, difficult airway, full stomach, or for intramuscular use when a suitable vein is inaccessible.
CONTRAINDICATIONS
- In patients with skeletal muscle myopathies
- In patients with known hypersensitivity to succinylcholine
- After the acute phase of injury following major burns, multiple trauma, extensive denervation of skeletal muscle, or upper motor neuron injury
- In patients with a personal or familial history or suspected genetic susceptibility to malignant hyperthermia
WARNINGS AND PRECAUTIONS
- Anaphylaxis: Severe anaphylactic reactions to neuromuscular blocking agents, including succinylcholine, have been reported. Some cases have been life-threatening and fatal. Take necessary precautions, such as the immediate availability of appropriate emergency.
- Risk of Death due Medication Errors: Unintended administration of Succinylcholine Chloride Injection may result in paralysis, respiratory arrest and death. Confirm proper selection of intended product and avoid confusion with other injectable solutions that are present in critical care and other clinical settings.
- Hyperkalemia: Succinylcholine Chloride Injection may induce serious cardiac arrhythmias or cardiac arrest due to hyperkalemia.
- Malignant Hyperthermia: Malignant hyperthermia may occur, especially in individuals with known or suspected susceptibility based on genetic factors or family history. Discontinue triggering agents, administer intravenous dantrolene sodium, and apply supportive therapies.
- Bradycardia: Intravenous bolus administration may result in profound bradycardia or, rarely asystole. The incidence is higher following a second dose of succinylcholine. Pretreatment with anticholinergic agents (e.g. atropine) may reduce the occurrence of bradyarrhythmias.
ADVERSE REACTIONS
Adverse reactions reported with succinylcholine are cardiac arrest, malignant hyperthermia, arrhythmias, bradycardia, tachycardia, hypertension, hypotension, hyperkalemia, prolonged respiratory depression or apnea.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or
www.fda.gov/medwatch.
DRUG INTERACTIONS
Drugs that May Enhance the Neuromuscular Blocking Action of Succinylcholine: promazine, oxytocin, aprotinin, certain non-penicillin antibiotics, quinidine, β-adrenergic blockers, procainamide, lidocaine, trimethaphan, lithium carbonate, magnesium salts, quinine, chloroquine, isoflurane, desflurane, metoclopramide, terbutaline, and drugs that reduce plasma cholinesterase activity.
INDICATIONS AND USE
Succinylcholine Chloride Injection is a depolarizing neuromuscular blocker indicated in adults and pediatric patients:
- As an adjunct to general anesthesia
- To facilitate tracheal intubation
- To provide skeletal muscle relaxation during surgery or mechanical ventilation
This Important Safety Information does not include all the information needed to use SUCCINYLCHOLINE CHLORIDE INJECTION, USP safely and effectively. Please see full prescribing information, including BOXED WARNING, for SUCCINYLCHOLINE CHLORIDE INJECTION, USP 100 mg per 5 mL and 200 mg per 10 mL.